
The most common adverse reactions in ≥20% of patients receiving nivolumab in combination with ipilimumab and platinum-doublet chemotherapy were fatigue, musculoskeletal pain, nausea, diarrhea, rash, decreased appetite, constipation, and pruritus. Median response duration was 10 months in the nivolumab plus ipilimumab and chemotherapy arm, and 5.1 months in the chemotherapy arm. Confirmed overall response rate (ORR) per BICR was 38% (95% CI: 33, 43) and 25% (95% CI: 21, 30) respectively. Median progression-free survival (PFS) per blinded independent central review (BICR) was 6.8 months (95% CI: 5.6, 7.7) in the nivolumab plus ipilimumab and chemotherapy arm and 5 months (95% CI: 4.3, 5.6) in the chemotherapy arm (HR 0.70 95% CI: 0.57, 0.86).
CHECKMATE 9LA CLINICAL STUDY TRIAL
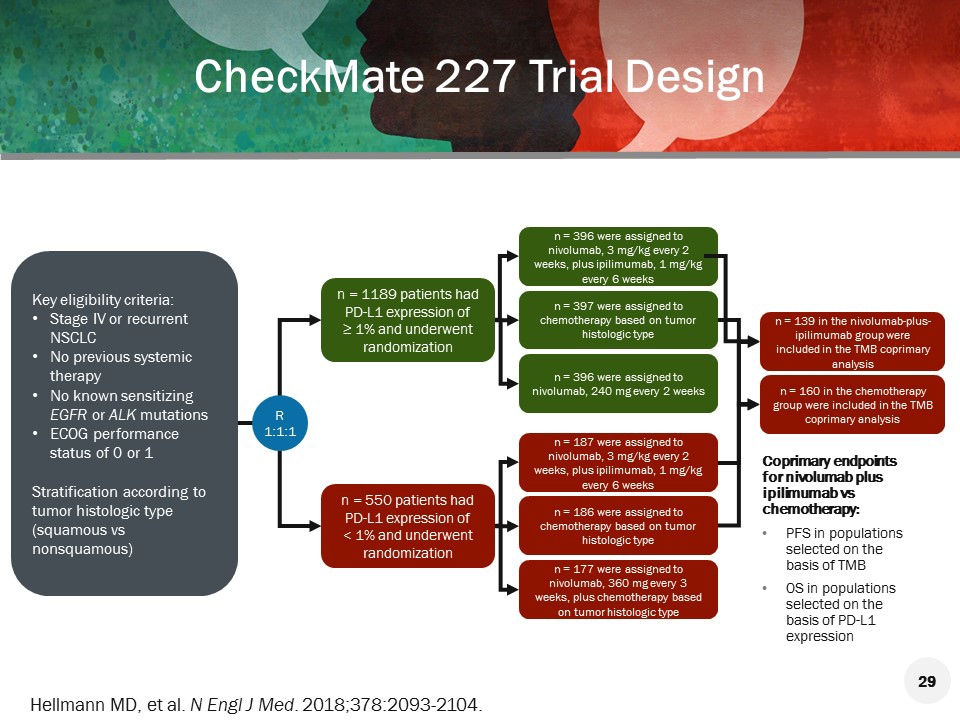
The trial demonstrated a statistically significant benefit in overall survival (OS) for patients treated with nivolumab plus ipilimumab plus chemotherapy compared to those who received chemotherapy. Patients were randomized to receive either the combination of nivolumab plus ipilimumab and 2 cycles of platinum-doublet chemotherapy (n=361) or platinum-doublet chemotherapy for 4 cycles (n=358).
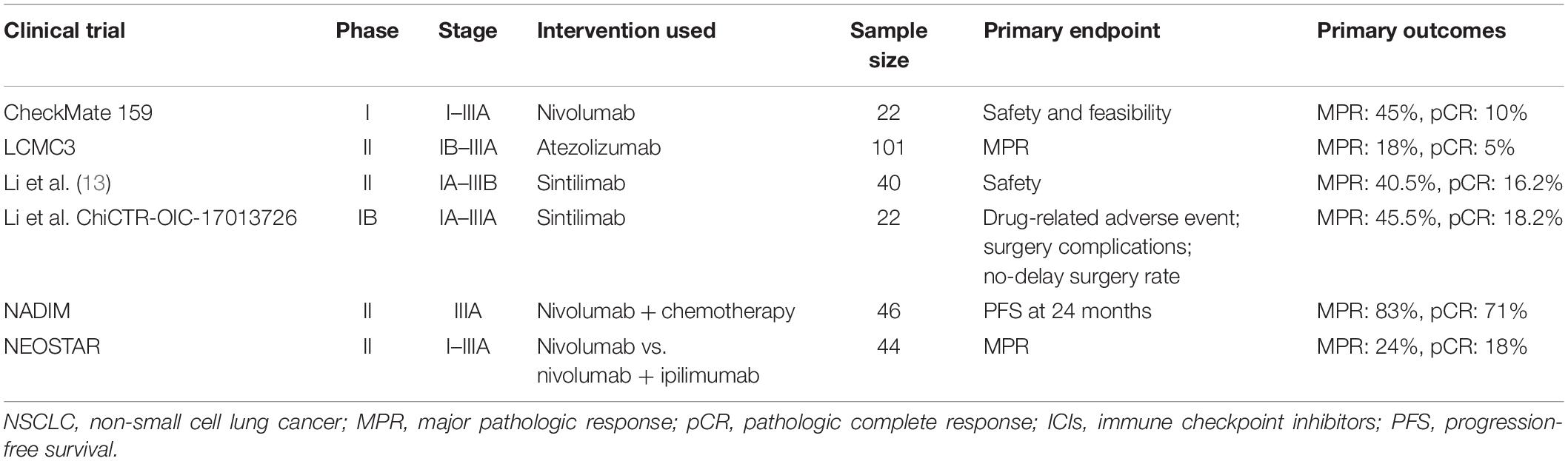
On May 26, 2020, the Food and Drug Administration approved the combination of nivolumab (OPDIVO, Bristol-Myers Squibb Co.) plus ipilimumab (YERVOY, Bristol-Myers Squibb Co.) and 2 cycles of platinum-doublet chemotherapy as first-line treatment for patients with metastatic or recurrent non-small cell lung cancer (NSCLC), with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrations.Įfficacy was investigated in CHECKMATE-9LA (NCT03215706), a randomized, open-label trial in patients with metastatic or recurrent NSCLC.


 0 kommentar(er)
0 kommentar(er)
